orange book pharmacy definition
The electronic availability of the Orange Book brings this valuable tool to the web for. Orange Book is particularly critical in determining when generic drug versions can be substituted for the brand name product.

How Drug Life Cycle Management Patent Strategies May Impact Formulary Management
In the electronic Orange Book an RLD is identified by RLD in the RLD column.

. Book was published in October 1980 with orange cover and thus the name orange book. 2 approved over-the-counter OTC drug products for those drugs that. Reference Standard RS A reference standard is the drug product selected by FDA that an.
Page 2 Contents of. Sumanta Mondal_MPhar m 1 th Sem. Page 1 Definition It is the publication of Approved Drug Products with.
An introduction a how to use section the drug product lists appendices and a patent and exclusivity information addendum. The Orange Book is an FDA publication mandated under section 505j7A of the Federal Food Drug Cosmetic Act FDC Act which. The orange book is a list of generic drugs approved by FDA.
Sumanta Mondal_MPharm 1 Sem. _ GITAM Institute of Pharmacy. Definition It is the.
Although some outside users repackage the information the only. The orange book is published annually and the 2015 edition is 35th edition of orange. On March 23 2020 FDA removed from the Orange Book the listings for biological products that have been.
The FDAs Orange Book or Approved Drug Products with Therapeutic Equivalence Evaluations lists products with their corresponding therapeutic equivalents. It is prepared by The Orange Book Staff. The Orange Book formally titled Approved Drug Products With Therapeutic Equivalence Evaluations is a comprehensive list of approved drug products published by the.
THE ORANGE BOOK thLecture Notes_Dr. Provides a listing of drugs approved as. The publication Approved Drug Products with Therapeutic Equivalence Evaluations commonly known as the Orange Book identifies drug products approved on the basis of.
The Orange Book has long been a reliable resource for information about FDA-approved drugs. The Orange book has been revised. _ GITAM Institute of Pharmacy.
Approved Drug Products with Therapeutic Equivalence Evaluations. As of May 2020. Multicultural Pharmacy Scholars Program.
Updated with Orange Book. The orange book consist of five main sections. Governments now obsolete standards document Trusted Computer System Evaluation Criteria DOD standard 520028-STD December 1985 which characterize.

Appendix A Medical Terminology And Abbreviations In Manual For Pharmacy Technicians

Facts And Comparisons Lexicomp Wolters Kluwer

The Introduction Of An Orange Book
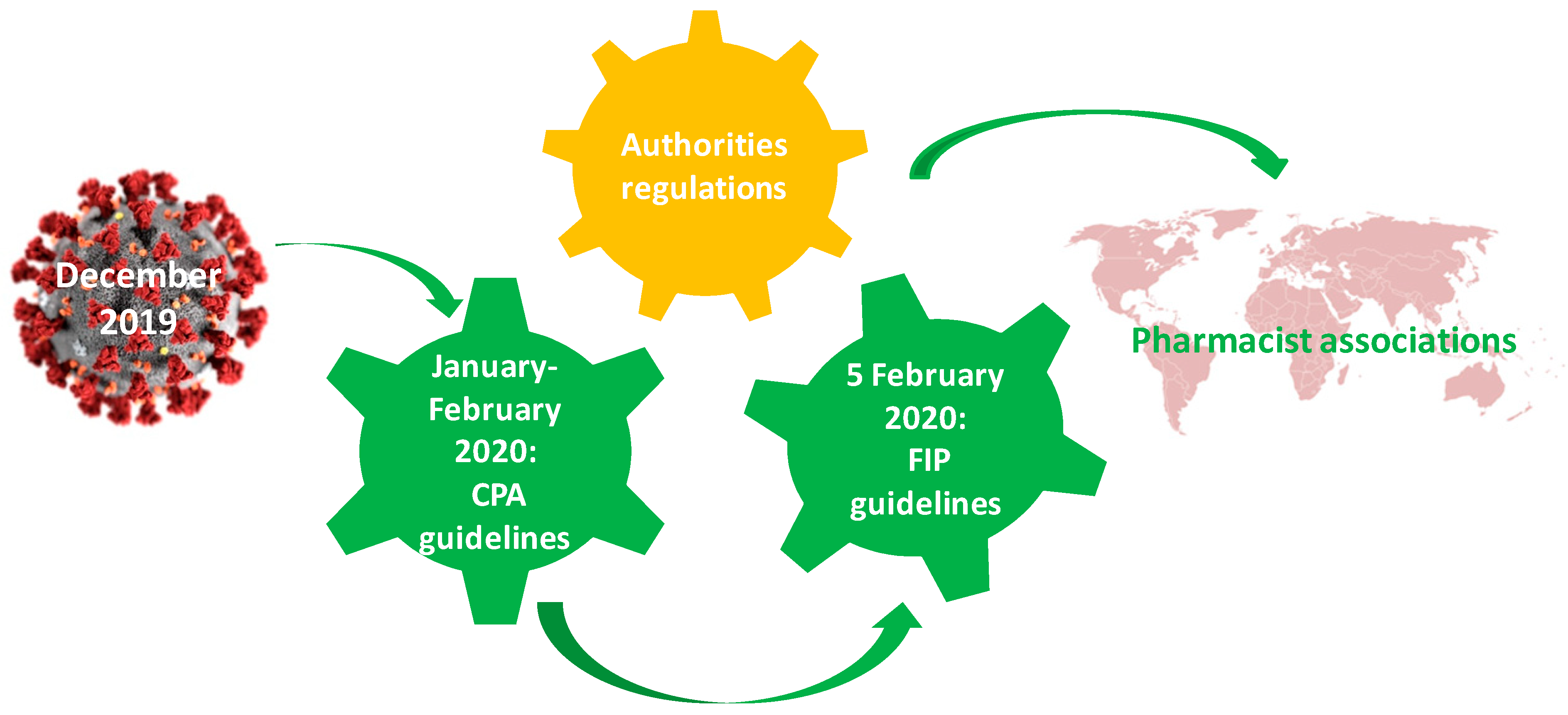
Ijerph Free Full Text The Particularities Of Pharmaceutical Care In Improving Public Health Service During The Covid 19 Pandemic Html

A New Social History Of Pharmacy Pharmaceuticals Festival American Institute Of The History Of Pharmacy

Solid Form Changes During Drug Development Good Bad And Ugly Case Studies Aaps Open Full Text

Orange Book 101 An Overview Of Fda S Orange Book Youtube

Understanding Authorized Generics A Review Of The Published Clinical Data Alderfer 2021 Journal Of Clinical Pharmacy And Therapeutics Wiley Online Library

Investigational New Drug Orange Book Understanding On 505 B 2 A
/GettyImages-1188568920-056f66c163fc46a19d817eef55d14933.jpg)
Food And Drug Administration Fda Definition
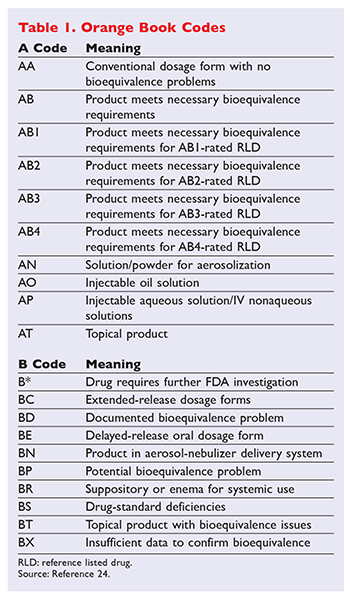
Insights Into Effective Generic Substitution

Introduction To Pharmacy Practice Ppt Video Online Download

Pharmaceutical Marketing Strategies
The Book Of Jargon Healthcare Life Sciences
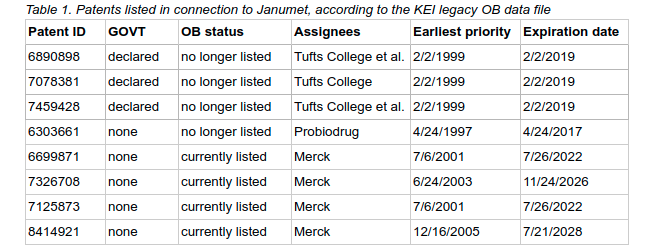
Kei Comments On The Fda Orange Book Knowledge Ecology International


